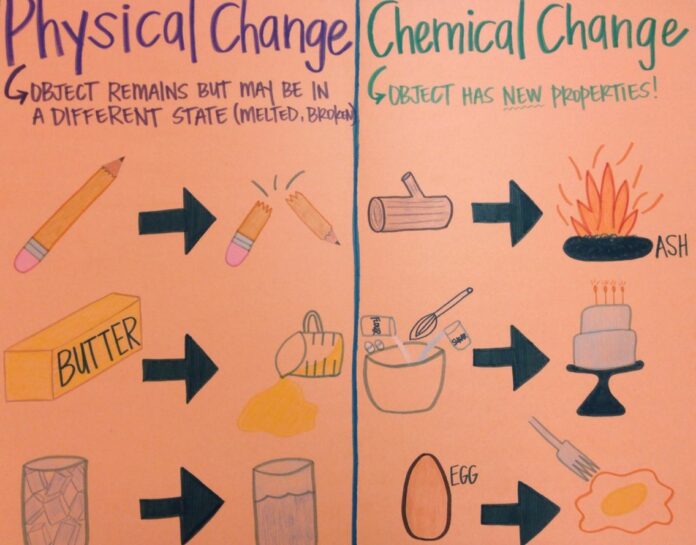
Chemical and physical changes for kids happen to surround you in regular daily existence. In any case, how might you let know if a substance has gone through a chemical or a physical change, and what is the distinction? So understanding the idea of chemical and physical changes is vital.
What is a Chemical Change of Matter?
A chemical change is a cycle where one sort of substance is changed into an alternate sort of substance. Chemical changes result in the formation of substances that did not exist when it was begun. It implies the changes in chemical change are long-lasting and irreversible.
For instance, when you consume a piece of paper, you can’t remake the paper from its remains. So this change is irreversible and long-lasting. Chemical changes occur on a subatomic level. This change happens when nuclear bonds are broken or made during chemical responses.
Chemical Change Examples
- Iron rusting
- Consuming kindling
- Natural matter
- decomposition
- Natural product ageing
- Processing food
- Broiling an egg
- Photosynthesis
- Ruined food
- Teeth rotting
- Breath (relaxing)
- Firecrackers detonating
- Souring of milk
Indications of Chemical Changes
Following are the potential indications of chemical change:
- Change in Color
- Change in Temperature
- Change in smell
- Change in taste
- Development of a Precipitate
- Development of Bubbles
Synopsis – Chemical Changes
In a chemical response, there is a change in the creation of the substances.
These sorts of changes produce substances that were not there when it was begun.
It occurs on a subatomic level.
Chemical changes produce new items.
Chemical change is irreversible and extremely durable.
It happens when nuclear bonds are broken or made during chemical responses.
What is a Physical Change of Matter?
Physical change is a cycle where a substance changes its condition of issue, yet chemical bonds are as yet unchanged. Physical changes include an item’s physical properties like size, variety, shape, and weight.
The changes in physical change are reversible and transitory. No new items are shaped during the physical change, and the atomic organisation continues as before.
For instance, when you liquefy an ice solid shape, it becomes fluid. Here the state is changed, however the water atoms (H2O) are as yet unchanged as ice 3D squares.
Physical Change Examples
- Freezing water to make ice
- Softening an ice 3D square
- Warming water to make steam
- A piece of paper cut into two pieces
- Bowing a paperclip
- Hacking vegetables
- Breaking a glass
- Cutting texture
Indications of Physical Changes
Following are the potential indications of physical change:
- Change of shape
- Change of state (strong, fluid, gas)
- Change in size
- Change in some other physical property
- Outline – Physical Changes
- The physical change influences just physical properties, i.e., shape, size, weight, and so on.
- In a physical change, there is no change in the structure of the substances.
- The atomic organisation continues as before.
- Physical changes don’t occur on a subatomic level.
- Physical changes don’t create new items.
- Physical change is reversible and impermanent.
What is Chemical Reaction?
A chemical response happens when at least one substance changes into various substances that have different physical and chemical properties. Connections between particles are broken and made to frame new atoms.
Reactant -A substance that goes into a chemical response.
Items – The substances created toward the finish of the response are known as the items.
For instance: Vinegar + Baking pop = Carbon dioxide.
Vinegar + Baking pop = Reactant
Carbon dioxide = Product
Sorts of Chemical Reactions
Mix Reaction – at least two basic substances join to frame a more perplexing one. A blend response is otherwise called a combination response.
A + B → AB
Deterioration Reaction – The course of a mind boggling substance being separated into more straightforward substances. Stomach muscle → A + B
Blend and deterioration responses are alternate extremes.
Burning Reaction – In this response, the substance responds with oxygen gas, delivering energy as light and intensity. Ignition responses should include Oxygen as one reactant.
A+B+Oxygen = Carbon dioxide + Water
Note: Product of burning is consistently water and carbon dioxide.
(FAQs)
Is bubbling water a chemical change?
No, bubbling water is definitely not a chemical change. It is a physical change on the grounds that the water fume actually has a similar subatomic construction as fluid water (H2O). Water bubbling, dissolving ice, tearing paper, freezing water and squashing a can are instances of physical changes.
Is consuming a chemical change?
Indeed, consuming is a chemical change.
Is rusting a chemical change?
Indeed, rusting is a chemical change
Is broiling an egg a chemical change?
Indeed, broiling an egg is a chemical change since it brings about the development of new particles. The intensity in the broiling system gives energy to the egg’s particles and achieves an extremely durable change in the substance.
What are the 5 signs of a chemical change?
These are the 5 signs of a chemical change:
1. Change in Temperature
2. Change in Colour
3. Change in smell
4. Change in taste
5. Development of a Precipitate