OSMOSIS EGG EXPERIMENT – While people read this, countless processes are taking over their bodies. Your stomach and smaller intestines are currently a liquid slurry through which the meal you recently ate is moving. To eliminate waste and surplus water, your kidney exerts much effort. Tears are secreted by lacrimal glands next to the eyes, allowing your eyelids to shut without endangering your eyeballs. What feature unifies these procedures in some way? They all depend on osmosis, water flow from one location to another.
In living organisms, the various solutions are typically divided by a selectively permeable membrane, such as renal tubules or cellular membranes. These function similarly to a net to retain solutes while allowing water to flow freely. Cells may retain the whole of their “guts” in this manner while yet exchanging water.
“osmosis egg experiment” plays a significant role throughout each of these functions and serves as a vital force in maintaining the health of each as well as every of your body’s cells. Without the need for a microscope, it is difficult to see osmosis. Yet if we use a shell-less chicken egg to make our version of a cell, we could observe what occurs when we change the osmotic equilibrium in the “cell”!
Materials:-
- 3 eggs
- three cups (large enough to fit the egg plus liquid)
- three butter knives
- clear vinegar (about 3 cups)
- Purified water (about 2 cups)
- (1 14 cups) of light corn syrup
- spoon with slots
- Cup for measuring (1 cup)
- spoons for measuring (1 tablespoon and 1/2 tablespoon)
- Marker and sticky notes Scale (optional)
Procedure:-
It’s safe to handle the egg in a lab, but wash your hands afterward to prevent unforeseen events! Afterward, an egg osmosis lab report is established.
1. Fill each glass with one egg. Add enough vinegar to coat each egg completely. The egg would begin to bubble to the surface and rise. Place a butter knife in a cup to support it down and keep it immersed.
2. Place the three glasses in the fridge and leave for 24 hours.
3. Pour off the old vinegar while keeping the egg in a lab firmly in the glass. Cover with new vinegar and stand for an additional 24 hours in the fridge. Continue this process until the membrane is left after the shells have completely disintegrated. It ought to take two to three days.
4. Start by removing the eggs with a slotted spoon, then rinse them inside the sink with running water. Additionally, wash the empty glasses.
5. Carefully place the shellless eggs on a plate and set them aside.
6. Make the following three separate sugar-water solutions, labeling them with sticky notes:
Glass 1: “Hypertonic” label. Corn syrup, one cup, should be added.
Glass 2: “Isotonic” logo on glass two. One cup of pure water should be added to the measuring cup after adding one and a half teaspoons of corn syrup.
Glass 3: label “hypotonic” on glass 3. Place 1 cup of pure water here. One egg without a shell should be carefully positioned within every glass. Place the glasses in the fridge for an additional day.
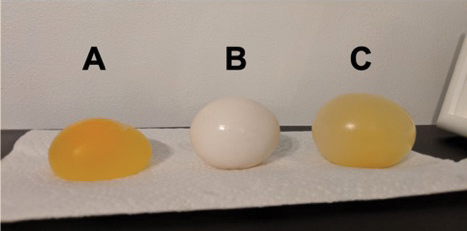
7. Take the glasses from the fridge first, then place the same eggs carefully on a plate. Weigh these eggs again and if you measured them before placing them within every solution. Every egg had a different fate; what was it?
READ MORE – WHAT IS THE BARWALA EGG RATE TODAY?
How does osmosis function?
The osmosis egg experiment is the scientific word for how liquid moves to various locations based on specific circumstances. In this instance, a concentration, or solutions with varying concentrations of soluble particles (solutes), governs the movement of water to various locations.
Solute concentration in both systems is equivalent because water always moves toward the location that has the greatest dissolved solutes. Imagine adding a drop of food coloring to a water container. Although if you don’t stir it, the food dye will eventually disintegrate into the water.
How did osmosis affect the size of the eggs, if at all?
For framing egg osmosis lab report, The outcomes ought to be as follows if the previous procedures are followed correctly. In the instance of the concentrated solution, the corn syrup contained more dissolved substances than the egg did. Water thus leaked from the egg further into corn syrup, which caused the egg to turn to mush.
There is no differential migration of solutes into or out of the egg in this isotonic solution since there were nearly equal amounts of solutes within the mixed corn syrup/water solution and the egg in a lab. It didn’t change in size.
Additional solutes were present in the egg compared to the distilled water in the hypertonic environment. Consequently, water entered the egg, growing it in size.
The Relationship Between Osmosis and You!
Each cell in the human body requires the required amount of water to maintain its structure, generate power, eliminate waste, and perform other essential bodily tasks.
This explains why properly balancing the concentration of solutes of administered medications with that of the patient’s bloodstream is necessary (i.e. isotonic). For instance, you might receive a 0.90% saltwater IV drip if you have been ill and started to feel dehydrated. Based on the number of dissolved salts in the water, your blood cells could shrivel and die or burst if the solution strayed too far from this threshold, rendering it no longer isotonic.
CONCLUSION
In conclusion, the article has attempted to give you information about the “osmosis egg experiment”. I hope you got a clear idea regarding the egg osmosis lab report.
FREQUENTLY ASKED QUESTIONS
Q1) what do eggs represent in the osmosis experiment?
Ans- Students will use an egg without the shell in this osmosis egg experiment. The egg without a shell would stand in for a cell and its membrane that is only semipermeable.
READ MORE – The Science Behind Scientific Method|